
A Dutch study suggests that CDK4/6 inhibitors can be administered as second-line therapy in patients with advanced hormone-dependent and HER2-negative breast cancer without decreased survival, but with fewer side effects. The scientists reported about it in the journal Nature.
Problem statement: immediately after diagnosis or only in the second line?
It is known that when used in combination with hormonal therapy, drugs that inhibit CDK4/6 (enzymes playing a major role in the process of cell division) improve treatment outcomes in hormone receptor-positive and HER2 receptor-negative breast malignancies. In principle, they can be used in both first- and second-line therapy.
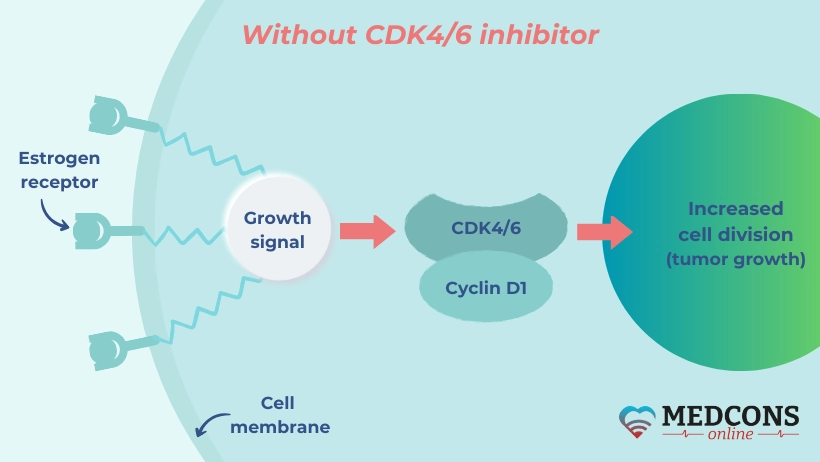
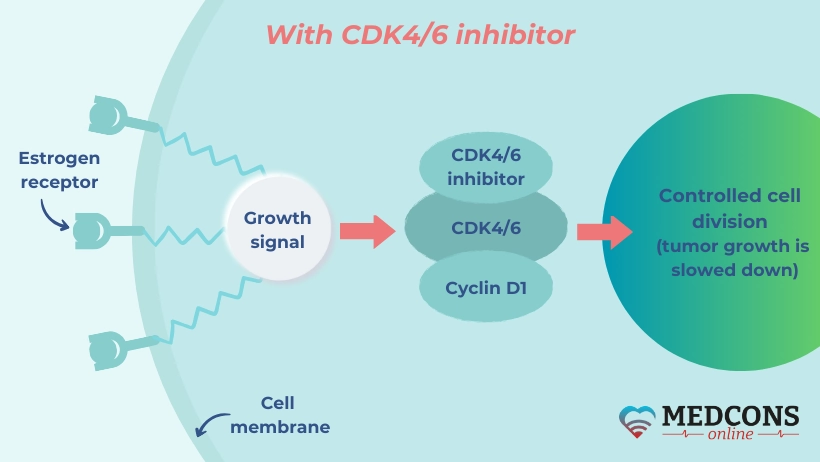
In the SONIA trial, a research team led by Gabe S. Sonke from the Department of Therapeutic Oncology at the Netherlands Cancer Institute in Amsterdam investigated whether one of the two time points was preferable.
About the trial
In the phase III trial, previously untreated patients received a CDK4/6 inhibitor either immediately after diagnosis of advanced breast cancer (first-line therapy, in combination with aromatase inhibitors) or after the failure of previous endocrine therapy (second-line therapy, plus fulvestrant, a cytostatic from the anti-estrogen group).
The mean follow-up period of 37.3 months showed no difference when the drug was used in first or second line. Progression-free survival was similar in both groups (median 31.0 vs. 26.8 months; p=0.10), as was the health-related quality of life. Exploratory analysis of overall survival also showed no differences in this regard.
Significant increase in treatment duration was associated with increased side effects
However, patients who received the CDK4/6 inhibitor in the first line used the drug for 16.5 months longer than patients who received it in the second line (median 24.6 vs. 8.1 months). Such a long duration of treatment did not bring additional benefits, but was accompanied by a 74% increase in side effects. While 2,763 grade ≥3 adverse events occurred with the first-line CDK4/6 inhibitor, 1,591 occurred with the second-line CDK4/6 inhibitor.
The researchers report that the longer duration of first-line CDK4/6 inhibitor treatment was also associated with significantly higher costs on the health care system. Based on price levels in the Netherlands, the difference amounted to about €30,000 per patient.
The study challenges the current standard
They add that current widespread recommendations for first-line use are not based on randomized data, but rather on concerns that patients are being deprived of effective therapy.
SONIA reaffirms that thorough studies examining sequencing options for therapy use are necessary to help understand how best to utilize the currently available drugs, and that this type of academic postmarketing research can be of great benefit to patients and the health care system.
References
- Sonke, G.S., van Ommen-Nijhof, A., Wortelboer, N. et al. Early versus deferred use of CDK4/6 inhibitors in advanced breast cancer. Nature 636, 474–480 (2024). https://doi.org/10.1038/s41586-024-08035-2

Comments — 0