
A large-scale study has shown that a drug approved for treatment of triple-negative breast cancer is also effective in metastasizing hormone-positive tumors.
About the drug
The medicine called sacituzumab govitecan (the brand name is Trodelvi) combines two active components: a monoclonal antibody (sacituzumab) and the cytotoxic substance govitecan.
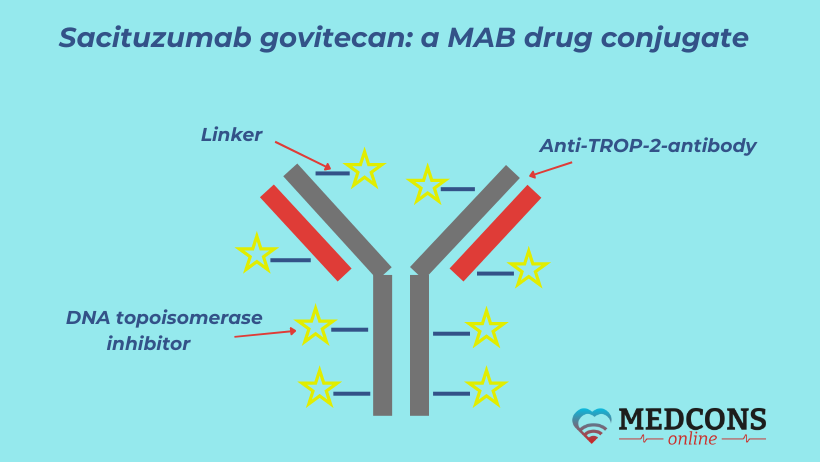
The first component is directed against the surface antigen of trophoblast 2 (Trop-2) cells. This is a glycoprotein (two-component protein) that is expressed by more than 90% of breast and bladder cancer cells and is involved in carcinogenesis and tumor progression. The second component is a cytostatic agent (a DNA topoisomerase inhibitor), its action is to stop DNA replication of tumor cells.
A recent study has expanded the scope of Trodelvie® use
At the 43rd Annual Congress of the German Society for Senology (DGS) in Dresden in June 2024, the results of the TROPiCS-02 study (a prospective, randomized trial involving 543 female patients with locally recurrent or metastasizing inoperable HR+/Her2- breast cancer) were released. All of them had previously received at least one line of therapy with an antihormonal drug, taxane, and a cyclin-dependent kinases 4 and 6 inhibitor (2 to 4 lines in total).
In TROPiCS-02, participants received sacituzumab govitecan or some other drug of the physician's choice.
The results for both parameters (progression-free survival and overall survival) were better with the two-component drug.
In the group of patients who had previously undergone 2 lines of therapy, the clinical benefit rate reached 41% compared to 25% in the control group. In the case of 3 lines of prior therapy it was 29 % compared to 20 %.
The incidence of grade 3/4 treatment-related adverse events was also independent of the number of prior lines of therapy.
Based on the results of the study, the researchers concluded: sacituzumab govitecan is effective for the treatment of metastatic breast cancer in hormone receptor-positive and HER2-negative patients regardless of the number of prior courses of therapy. It reduces the risk of recurrence by 39%.
References
- https://www.aerzteblatt.de/nachrichten/152790/Sacituzumab-Govitecan-verbessert-Prognose-beim-metastasierten-Brustkrebs?rt=33b3f3a8193547fd222fecc0138a4241
- https://www.aerzteblatt.de/nachrichten/134855/Brustkrebs-Sacituzumab-Govitecan-ist-moegliche-neue-Option-bei-inoperablen-intensiv-vorbehandelten-HR-Her2-Tumoren
- Hope S Rugo, MD †, Aditya Bardia, MD †, Frederik Marmé, MD,Javier Cortés, MD, Peter Schmid, MD, Delphine Loirat, MD et al.
- Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. The Lancet. Open AccessPublished:August 23, 2023DOI:https://doi.org/10.1016/S0140-6736(23)01245-X

Comments — 0