Patients with early-stage triple-negative breast cancer may benefit from supplementing chemotherapy with the administration of pembrolizumab. This was supported by the results of the phase 3 KEYNOTE-522 trial, published in the New England Journal of Medicine.
About the triple-negative breast cancer
Breast malignancies are divided into subtypes based on the number of estrogen, progesterone, and human epidermal growth factor receptors 2 (HER2) present on the surface of cancer cells. All three receptors are known to promote the growth of breast tumors. Therefore, drugs that block their activity are the mainstay of treatment for this cancer.
This situation is observed in about 15-20 % of all cases of this disease. It mainly occurs in young women and is associated with a high risk of metastasis and recurrence, which means a poor prognosis.
Methods
KEYNOTE-522 is a clinical trial involving patients with early-stage triple-negative high-risk breast cancer, funded by Merck Sharp & Dohme, the manufacturer of KEYTRUDA®, which is used to treat many types of cancer.
The active ingredient in the drug is pembrolizumab. It is a monoclonal antibody that blocks the PD-1 receptor. In a normal situation, PD-1 in interaction with its ligands counteract excessive immune reactions of the body.
Pembrolizumab inhibits the PD-1 receptor, blocking the ligands that would deactivate it and prevent an immune response. This allows the immune system to target and destroy cancer cells.
The phase 3 KEYNOTE-522 study involved previously untreated patients with triple-negative stage II or III breast cancer. Purpose: To investigate the efficacy of adding pembrolizumab to standard chemotherapy.
The participants were randomly divided into two groups which received different neoadjuvant and adjuvant therapies:
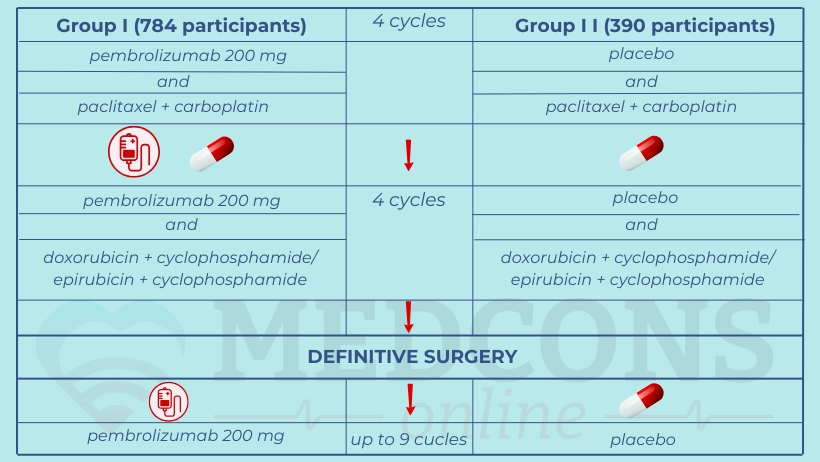
The primary endpoints (expected study outcome options) were complete pathological response (no residual tumor in the breast and lymph nodes) at the time of definitive surgery and event-free survival. Overall survival was the secondary endpoint.
Results and scientific conclusions
Of the 1,174 patients, 784 were randomized into the chemotherapy+pembrolizumab group and 390 to the chemotherapy+placebo group. At the time of data collection on March 22, 2024, the median duration of follow-up was 75.1 months.
60-month overall survival in the first group turned out to be 86.6% compared with 81.7% in the second group. Event-free survival in 5 years was also better with pembrolizumab (81.2% versus 72.2% without it). Side effects were consistent with the known safety profile of pembrolizumab and cytostatics.
The research team concluded,
References
- Peter Schmid, M.D., Javier Cortes, M.D., Rebecca Dent, M.D., Heather McArthur, M.D., Lajos Pusztai, M.D., Sherko Kümmel, M.D., Carsten Denkert, M.D., +16, for the KEYNOTE-522 Investigators*. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. Published September 15, 2024 N Engl J Med 2024;391:1981-1991. DOI: 10.1056/NEJMoa2409932 VOL. 391 NO. 21.
- Schmid P, Cortés J, Dent RA, et al: Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for high-risk early-stage TNBC: Overall survival results from the phase III KEYNOTE-522 study. ESMO Congress 2024.

Comments — 0