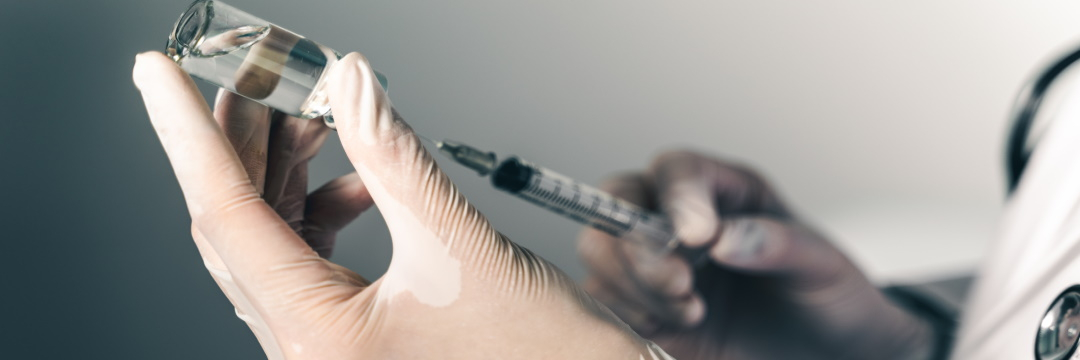
Breast cancer is the most common malignancy among women, accounting for about 30 percent of all cancerous conditions. The Robert Koch Institute estimates that one in eight women will develop mammary carcinoma in her lifetime. Only one percent of all newly diagnosed cases involve men.
And yet, although today the chances to be cured are good, they largely depend on the type and stage of the tumor.
Vaccination is the future
Scientists around the world are working hard to find new ways to better treat and improve the prognosis of this severe condition. One of their efforts is the search for a vaccine that can fight malignant cells.
And now the first such drug has already successfully passed the initial stage of clinical trials. This vaccine could be a breakthrough in the fight against the disease.
The first breast cancer vaccine was first successfully tested in women by scientists at the University of Washington Medical Center (UWSM) in Seattle lead by Dr. Nora Disis. The DNA-based drug showed good results in the tests, their results were reported in the specialized journal JAMA Oncology.
Scientists have devoted more than 20 years to finding a vaccine to fight breast cancer. The latest results of these human studies were very promising. They confirmed that the drug can induce a strong immune response directed against cancer cells.
What types of breast cancer are there?
Mammary gland cancer is not a homogenous disease. It falls into two different categories: there are hormone-sensitive tumors and there are tumors whose cells are characterized by increased expression of epidermal growth factor receptor HER2 (the so-called HER2 - positive). Four more subtypes are distinguished among these types.
The HER2 receptor is a protein that is present on the surface of many cells, but in some cases of breast cancer, it is 100 times more abundant than normal.
Therefore, HER2 - positive variants of the disease tend to be more aggressive and have an increased risk of recurring after treatment (relapse).
About 20 to 25 out of 100 breast carcinomas excessively express HER-2 receptors. It was their suppression by the body's immune response that was the focus of the search for a new vaccine, because even if it could help even these 20-25% of cases, it would mean a real breakthrough in oncology.
What were the first-phase clinical trial results?
The study involved 66 women who had the respective type of breast cancer (HER2-positive) which had spread to other organs (metastases). The patients were divided into three groups depending on the dose of the DNA-base vaccine administered to them (low, medium, and high).
The study participants underwent regular medical examinations in the interval from 3 to 13 years, during which time the development of the disease was monitored. It was found that the strongest immune response to the vaccine was observed in patients who received the medium dose of the medication.
The vaccine was found to prolong life in 80 percent of cases.
Even though the scientists' initial goals were not to find out whether the new drug slows down the progression of cancer or whether it can stop it altogether, it became clear during the research process, which lasted an average of 10 years, that the condition of patients who had received the vaccine was significantly better than that of patients with the same form and stage of cancer who had not undergone vaccination. Half of the women in this category died within five years despite treatment. In the group of patients who had been vaccinated, 80% were still alive after 10 years.
How does the new vaccine work?
The new vaccine is not really a prophylactic vaccine as it is usually understood and used for other diseases. In the first phase of the trial, injections were given exclusively to patients who already had breast cancer.
The drug is a DNA-based active ingredient that specifically targets the HER2 receptors that cause accelerated growth of malignant cells. It contains the genetic information to build antibodies that specifically attach to the HER2 - fixation site. Under the influence of the inoculation, a protein begins to be produced that kills the diseased cells.
Scientists reasonably hope that the new vaccine will train the immune system to attack abnormal cells without affecting healthy cells. Theoretically, the authors believe, such a vaccine could also be used to treat other cancers that are characterized by the presence of this protein.
The study's most important finding: the vaccine is safe and does not cause serious consequences
The most common reactions, which occurred in exactly half of the patients, were similar to those seen with the COVID-19 vaccine: redness and swelling at the injection site, occasional mild fever, and flu-like symptoms. The desired immune response was observed in those patients who received a medium dose of the vaccine.
What do the trial results mean?
The results obtained by scientists in Phase I clinical trials of the new DNA-based substance give reasonable hope that a turning point in cancer vaccine development has now been reached. Phase II clinical trials of the promising drug are now underway.
The drug developers’ effort has already been awarded the famous American Internet portal Gizmodo Science Fair, dedicated to science and new technologies.
At the same time, researchers are testing two other "candidates”. Similar drugs against ovarian, colon, lung, bladder, and prostate carcinoma are also in development.
If the outcome is as positive after Phase II trials of the breast cancer vaccine, the next and final stage of the study could begin. However, it may take some time before the substance is allowed for commercial use. Scientists estimate that the breast cancer “inoculation” drug could appear on the market within the next five years.
The experts are optimistic and hope that one day the vaccine could be used for other types of mammary carcinoma.
References
- Safety and Outcomes of a Plasmid DNA Vaccine Encoding the ERBB2 Intracellular Domain in Patients With Advanced-Stage ERBB2-Positive Breast Cancer. A Phase 1 Nonrandomized Clinical Trial
- Mary L. (Nora) Disis, MD1; Katherine A. Guthrie, PhD2; Ying Liu, PhD1; et al.
- AMA Oncol. 2023;9(1):71-78. doi:10.1001/jamaoncol.2022.5143

Comments — 1
Валентина
Если это всё действительно так, то скорей бы уже! Очень ждём.